Combination of Interscalene Brachial Plexus Block with General Anesthesia Attenuates Stress and Inflammatory Response in Arthroscopic Shoulder Surgery
1Department of Anesthesiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, Guangdong, China
2Department of Anesthesiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
3Department of Anesthesiology, University of Virginia, Charlottesville, VA 22908, USA
aThese authors contributed equally to this work.
*Correspondence to: Mingyan Guo, E-mail: guomyan@mail.sysu.edu.cn; Fei Wang, E-mail: wangf23@mail.sysu.edu.cn
Received: April 28 2021; Revised: June 4 2021; Accepted: June 16 2021; Published Online: July 2 2021
Cite this paper:
Daowei Lin, Zhixiao Han, Yanni Fu, Xiaoqiu Zhu, Jin Li, Hui Xu, Jing Wen, Fei Wang and Mingyan Guo. Combination of Interscalene Brachial Plexus Block with General Anesthesia Attenuates Stress and Inflammatory Response in Arthroscopic Shoulder Surgery. BIO Integration 2021; 2(4): 161–168.
DOI: 10.15212/bioi-2021-0013. Available at: https://bio-integration.org/
Download citation
© 2021 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
In arthroscopic shoulder surgery, general anesthesia (GA) is the common method of anesthesia. Recently, the combined usage of interscalene brachial plexus block with general anesthesia (ISB/GA) was reported to have a lower incidence of adverse side effects than GA alone. However, to date, no study has compared stress and inflammatory responses between these two methods. Since stress and inflammatory responses are critical on intraoperative management and postoperative recovery, we integrated the laboratory and clinical methods and compared the stress and inflammatory factors, such as epinephrine, norepinephrine, glucose, lactate, inflammatory factors tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), as well as the clinical outcomes to determine whether ISB/GA provides an advantage on stress and inflammatory inhibition. Data showed that ISB/GA resulted in significantly lower epinephrine, norepinephrine, and glucose levels perioperatively. Six hours after operation, the TNF-α and IL-6 levels were also significantly lower in the ISB/GA group. ISB/GA patients had lower blood pressure, a more stable heart rate, lower visual analog scale score, and less opioid consumption during and after surgery. Our results indicate that ISB/GA is a better choice for arthroscopic shoulder surgery, owing to less stress and inflammatory responses during and after operation, which provides better clinical outcomes. Therefore, we recommend ISB/GA as a preferred anesthesia method for arthroscopic shoulder surgery.
Keywords
Arthroscopic shoulder surgery, general anesthesia, inflammatory response, interscalene brachial plexus block, stress response.
Introduction
Arthroscopic shoulder surgery is usually performed under general anesthesia (GA) or interscalene brachial plexus block (ISB). The results of the studies of Brown et al. [1] and Arciero et al. [2] demonstrated that ISB have several benefits for this type of surgery over GA. ISB provided great intraoperative muscle relaxation and had fewer postoperative side effects as well as shorter duration of hospitalization [1, 2]. However, arthroscopic shoulder surgery is usually accompanied by acromial decompression or rotator cuff repair, with extracapsular irrigation. The occurrence of extravasation of irrigation fluid around the shoulder, chest wall, and trachea could lead to the compression of the chest cavity and upper airway [3]. Thus, ISB in combination with general anesthesia (ISB/GA) was considered a proper anesthesia alternative in arthroscopic shoulder surgery in order to achieve better clinical outcomes [4, 5]. The results of a meta-analysis of the safety and efficacy of ISB/GA in comparison to GA only indicated that patients undergoing arthroscopic shoulder surgery with ISB had lower intraoperative blood pressure and heart rate (HR), shorter extubation time, general lower pain scores, and lower incidence of adverse events than with GA only [6].
Surgery usually generates stress and inflammatory responses, a complex process involving multiple system throughout the body, facilitated by a combination of immunologic, metabolic, and endocrine systems [7]. These responses vary depending on severity of tissue injury and duration of surgery, making them a double-edged sword. Moderate stress is a normal defense reaction to protect the body from harm; however, excessive stress has the risk of stimulating cytokine production: elevated catecholamine leads to hemodynamic fluctuations; high metabolic blood sugar increase perioperative wound infection; changes in immunity increase the possibility of leukocyte infiltration in the wound [8, 9]. These, in turn, result in postoperative organ dysfunction, increased length of hospital stay, and mortality [10].
To date, few studies were done to compare stress and inflammatory responses comparing ISB/GA with GA. In this study, we aimed to elucidate whether the combination of ISB/GA could result in reduced inflammatory and stress response compared with GA in arthroscopic shoulder surgery.
Materials and methods
Ethical statement and trial registration
This is a prospective randomized endpoint study conducted according to the Declaration of Helsinki. The study protocol was approved by the ethical committee of Sun Yat-sen Memorial Hospital, Guangzhou, China (protocol number: SYSEC-KY-KS-2015-026). All participants were properly informed of the study and were asked to provide informed consent prior to enrollment. This study was registered in the Chinese Clinical Trial Registry Service (http://www.chictr.org.cn) under clinical trial number ChiCTR-INR-17010912.
Sample size calculation
The sample size was calculated based on the difference of perioperative stress and inflammatory factors. According to the study of Sağlık et al. [11], there was a significant difference in glucose level at 2 hours after operation in patients under GA and those under epidural and GA, with mean values of 143.8±18.6 and 120±10.3 mg/dL, respectively. As there were five more markers (lactate, epinephrine, norepinephrine, tumor necrosis factor α [TNF-α], interleukin 6 [IL-6]) to evaluate aside from glucose, more cases were required. We defined the null hypothesis as when the population means of the experimental and control groups were equal to a power of 0.99. Type I error probability was set to 0.005. Using PASS 15 (NCSS, Kaysville, UT, USA), we confirmed that 24 subjects were required for each group. Furthermore, as the observative endpoint was evaluated within 48 hours after operation, we assumed a 20% dropout rate for our study. Therefore, 30 subjects were required for each group.
Patients and treatments
Participants were all recruited from the Orthopedics Department of Sun Yat-sen Memorial Hospital. Eighty-four participants (ages 18–60 years) who were scheduled for arthroscopic rotator cuff surgery were included in the study. Patients whose conditions met American Society of Anesthesiologists (ASA) physical status I and II were included in this study. The exclusion criteria were preexisting medical problems such as respiratory or cardiac disease, diabetes, and/or preexisting coagulopathy; hypersensitivity to local anesthetic; patients with simple debridement or partial repair; a reoperation due to a retear; an open or mini-open repair; repair of the subscapularis; a previously operated lesion; or any other associated lesion and inflammatory joint diseases. Finally, 60 participants were included and randomized into two groups using a computerized random-number generator: the GA group (n=30) and the ISB/GA group (n=30). All surgeries were performed by the same surgeon.
In the ISB/GA group, patients received 10 mL of 2% lidocaine and 10 mL of 1% ropivacaine compound solution. The local anesthetic was delivered via Stimuplex 22-gauge puncture needle (B. Braun, Melsungen, Germany) with real-time ultrasound guidance. Thirty minutes later, the loss of sensation in the shoulder and upper arm were confirmed, indicating successful brachial plexus block. Participants were excluded for further analysis if the nerve block was unsuccessfully established. After the procedures, general anesthesia induction was conducted for all patients with 2 mg/kg of propofol (AstraZeneca SPA, Macclesfield, UK), 3 μg/kg of fentanyl (Yichang Humanwell Pharmaceutical Co., Ltd, Yichang, China), and 0.2 mg/kg of cisatracurium besylate (Jiangsu Hengrui Medicine Co., Ltd, Jiangsu, China). Then endotracheal intubation was performed, and controlled ventilation was used for continuous respiration. Anesthesia was maintained with sevoflurane inhalation and remifentanil intravenous administration if needed. In the GA group, patients received the same general anesthesia as that of the ISB/GA group, but without no SB. In both groups, cisatracurium were supplemented as necessary to provide adequate muscle relaxation needed during the surgery. The remifentanil consumption of patients from both groups were recorded for further analysis.
For all participants in both groups, the end-tidal carbon dioxide (ETCO2) was maintained between 35 and 45 mmHg and the Narcotrend Index value was maintained at 36–56 (stage D1–E0) by adjusting the concentration of sevoflurane. During the surgery, the patients’ body temperature was maintained around 36°C with a forced air-warming blanket. An arterial catheter was placed in the radial artery of the contralateral arm for continuous pressure monitoring. The mean arterial blood pressure (MAP) was maintained within 55 ± 5 mmHg by adjusting the sevoflurane inhalation and remifentanil infusion during the surgical procedure to provide clear surgical field of vision. Phenylephrine or nicardipine was applied when refractory hypotension or hypertension occurred, and the dose of the drugs was recorded. In addition, when the HR was <50 or >100 bpm, atropine or esmolol was used, respectively, and the dose of each administration was also recorded.
All patients were received arthroscopic shoulder cuff repair in a semi-reclining position with an elevated upper limb. Throughout the surgery, 0.9% normal saline was continuously injected to ensure a clear vision of the surgical field. Anesthetics administration was terminated when the surgery was completed (i.e., when the wound was closed and the patient was returned to supine position). After surgery, the endotracheal tube was removed when patients were able to follow verbal commands to open their eyes. Patient-controlled analgesia with fentanyl was administered after the surgery. Fentanyl consumption and their side effects were recorded for further analysis. The visual analog scale (VAS) score, which was the standardized score used to assess the patient’s pain level, was performed before any anesthesia procedure (baseline) and at 1, 6, 12, 24, and 48 hours postoperation. The duration of anesthesia (from anesthesia induction to tracheal extubation), surgical duration (from skin incision to wound closure), time to eye opening (from anesthetic termination to eye opening) and other pronounced side effects, such as agitation, nausea, and vomiting, were also recorded.
Analysis of biomarkers
To analyze inflammatory and stress response, blood samples were collected from all participants in both groups for the measurement of the plasma levels of stress factors epinephrine, norepinephrine, glucose, lactate, and inflammatory factors TNF-α and IL-6 at six time points: before anesthesia (T0); 5 min after arthroscopy insertion (T1); 1 hour after arthroscopy insertion (T2); at surgery completion (T3); 1 hour after surgery completion (T4); 6 hours after surgery completion (T5). The blood samples were centrifuged to separate plasma; then, the plasma was stored at –80°C until further analysis.
The stress and inflammatory factors were assayed in the biochemical laboratory using ELISA kits (Quantikine; R&D System Inc., Minneapolis, MN, USA) to assess the concentrations of TNF-α and IL-6. Briefly, reference standards and samples were placed into respective wells coated with monoclonal antibodies specific for each cytokine. The plates were then washed five times to remove nonadherent materials. Then, enzyme-linked secondary antibodies specific for each primary antibody were added to each well. The plates were washed again, followed by the addition of substrate solution and amplifier solution. The stop solution was finally added to the wells and absorbance was measured by a plate reader (SpectraMax 190 ELISA Reader, Molecular Devices, Shanghai, China). The plasma concentrations of lactate and glucose were determined using enzymatic kits (Boehringer Mannheim GmbH, Mannheim, Germany). To analyze the concentration of epinephrine and norepinephrine, blood was collected in lithium-heparinate tube containing 10 mL/L of a solution of 76 g/L EGTA and 61 g/L glutathione for stabilization and were quantified by reversed phase high-performance liquid chromatography with electrochemical detection (Chromakon 500; Kontron, Eiching, Germany).
Statistical analysis
Continuous parameters, including age, body mass index (BMI), anesthesia and surgery durations, biomarker levels, intraoperative remifentanil and postoperative fentanyl consumption, and VAS scores, are presented as mean ± standard error of mean (SEM) or mean ± standard deviation (SD). Categorical factors, such as sex and whether participants showed agitation, are presented as numbers and percentage (%). Differences between the two groups were tested with χ2 test for categorical variables and t-test for continuous variables. Differences among various time points were tested with one-way analysis of variance (ANOVA). Because the two groups had equal numbers of participants, the Bonferroni method was used for post hoc multiple comparisons of heterogeneity of variance, and Dunnett’s test was used to determine homogeneity of variance. The χ2 test, t-test, and ANOVA were performed by GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). P<0.05 was considered as the significance level.
Results
In this study, we compared the stress and inflammatory response between the ISB/GA and GA groups and found that ISB can significantly decrease the plasma levels of stress and inflammatory factors, such as epinephrine, norepinephrine, glucose, lactate, TNF-α, and IL-6. Moreover, ISB could significantly reduce opioid consumption during the operation and provide better pain relief postoperation.
Participants
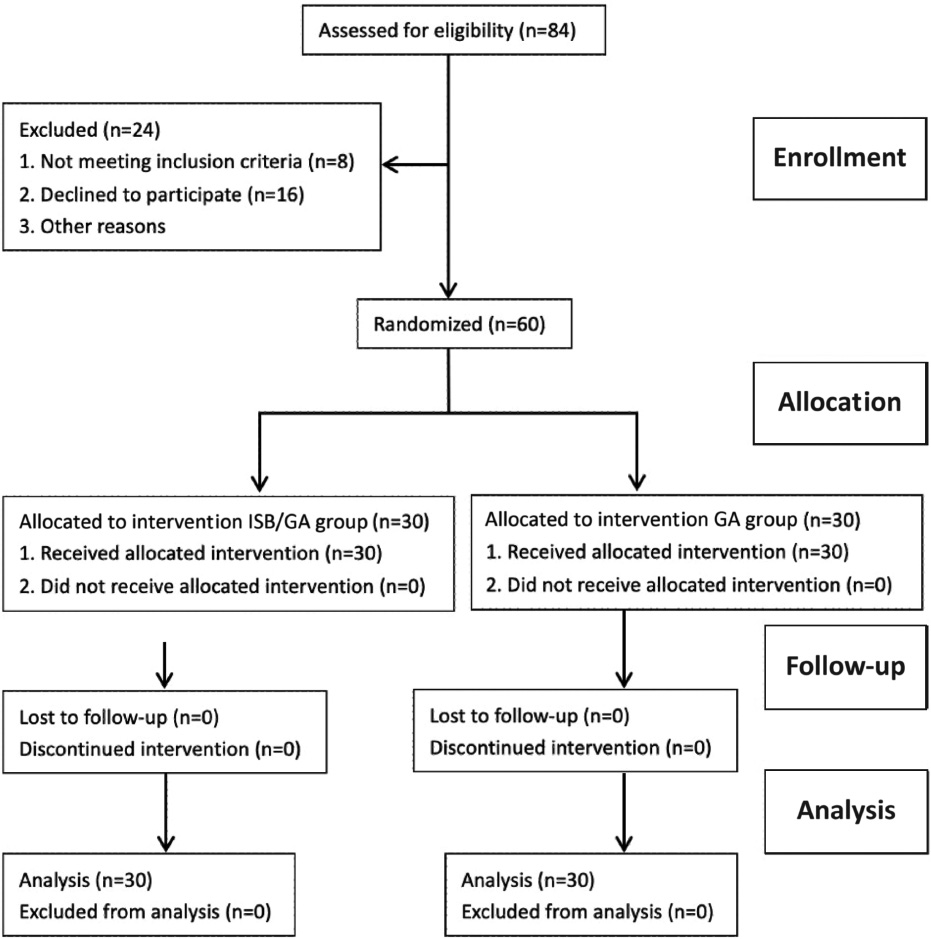
Eighty-four patients were scanned for study eligibility. Among them, 8 patients were excluded due to diabetes (n=1), hypertension (n=2), and inclusion of other injuries (n=5). The remaining 76 patients were eligible for participating in this study. Of these, 16 declined, and a total of 60 patients were included in the study and were required to provide written informed consent. The Consolidated Standards of Reporting Trials participant diagram for this study is given in Figure 1. All 60 enrolled participants were randomized into two groups (ISB/GA group, n = 30 patients; GA group, n = 30 patients). The interscalene brachial plexus was identified by guidance of ultrasound for all patients, and block success was confirmed before general anesthesia induction (Figure 1).
Figure 1 Consolidated standards of reporting trials (CONSORT) flow diagram. ISB/GA: brachial plexus block in combination with general anesthesia; GA: general anesthesia.
The demographic characteristics and baseline of the participants are shown in Table 1. Patients in both groups have similar age, sex ratio, weight, height, and BMI. All participants had rotator cuff repair under shoulder arthroscopy. The period of anesthesia or surgery and volume of infusion and irrigation fluid did not significantly differ between the two groups.
Table 1 Characteristic and Operation Details of Patients
| GA Group | ISB/GA Group | P | |
|---|---|---|---|
| Age (years)a | 37.8±12.0 | 40.4±14.1 | 0.528 |
| Sex (female/male)b | 9/21 | 8/22 | 0.84 |
| Weight (kg)a | 60.0±10.2 | 58.5±10.3 | 0.654 |
| Height (cm)a | 166.6±9.4 | 163.2±10.5 | 0.271 |
| BMIa | 21.5±2.3 | 21.9±2.6 | 0.583 |
| Duration of anesthesia (min)a | 138.6±28.8 | 147.6±30.9 | 0.678 |
| Duration of surgery (min)a | 99.5±29.2 | 103.3±29.8 | 0.332 |
| Infusion fluid volume (mL)a | 833.3±329. | 952.4±350.2. | 0.269 |
| Irrigation fluid volume (mL)a | 17428.6±3091.5 | 16714.3±3084.5 | 0.377 |
Values are means±SD or number. GA: general anesthesia; ISB/GA: brachial plexus block in combination with general anesthesia. aPerformed with t-test; bPerformed with χ2 test.
Plasma stress factors analysis
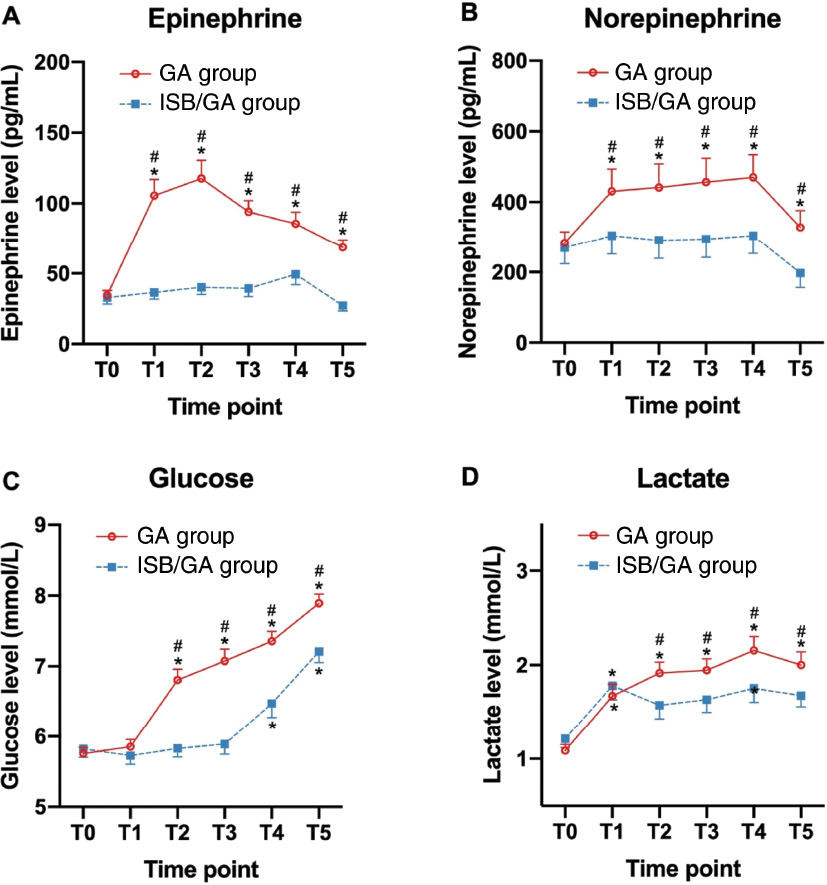
The epinephrine, norepinephrine, glucose, and lactate levels were similar at baseline level (ISB/GA group vs. GA group: epinephrine, 33.0 vs. 34.5 pg/mL; norepinephrine, 269.7 vs. 284.1 pg/mL; glucose, 5.8 vs. 5.8 mmol/L; lactate, 1.2 vs. 1.1 mmol/L; no statistical significance between the two groups). At T1, the epinephrine and norepinephrine values were significantly increased in the GA group and were significantly higher than those of the ISB/GA group (ISB/GA group vs. GA group: epinephrine, 36.6 vs. 105.6 pg/mL; norepinephrine, 304.5 vs. 434.1 pg/mL); the lactate level was significantly elevated in both groups; no significant change in glucose level was observed in either group (ISB/GA group vs. GA group: glucose, 5.7 vs. 5.9 mmol/L; lactate, 1.8 vs. 1.7 mmol/L). At T2 to T5, the epinephrine, norepinephrine, glucose, and lactate levels showed an increase trend in both groups. In the GA group, the epinephrine levels (ISB/GA group vs. GA group: T2, 40.2 vs. 117.7 pg/mL; T3, 39.4 vs. 94.1 pg/mL; T4, 49.4 vs. 85.6 pg/mL; T5, 27.2 vs. 68.5 pg/mL), norepinephrine (ISB/GA group vs. GA group: T2, 291.7 vs. 441.8 pg/mL; T3, 295.2 vs. 457.3 pg/mL; T4, 304.7 vs. 470.3 pg/mL; T5, 197.8 vs. 328.5 pg/mL), glucose (ISB/GA group vs. GA group: T2, 5.8 vs. 6.8 mmol/L; T3, 5.9 vs. 7.1 mmol/L, T4, 6.5 vs. 7.4 mmol/L; T5, 7.2 vs. 7.9 mmol/L), and lactate (ISB/GA group vs. GA group: T2, 1.6 vs. 1.9 mmol/L; T3, 1.6 vs. 1.9 mmol/L, T4, 1.8 vs. 2.2 mmol/L; T5, 1.7 vs. 2.0 mmol/L) significantly increased than the baseline level, whereas the epinephrine and norepinephrine levels at T2 to T5 and the glucose levels at T2 to T3 were not significantly elevated in the ISB/GA group. The levels of these four factors were all significantly higher in the GA group than those in the ISB/GA group (Figure 2). The results indicated that ISB/GA could significantly attenuate stress response compared to GA only.
Figure 2 Perioperative plasma stress factors between ISB/GA and GA. (A) Plasma levels of epinephrine; (B) Plasma levels of norepinephrine; (C) Plasma levels of glucose; (D) Plasma levels of lactate. ISB/GA: brachial plexus block combined general anesthesia; GA: general anesthesia. T0 = baseline; T1 = 5 min after arthroscopy insertion; T2 = 1 hour after the beginning of surgery; T3 = at the time of wound closure; T4 = 1 hour after surgery; T5 = 6 hours after surgery. Data are expressed as mean ± SEM. *P < 0.05 compared with T0; #P < 0.05 compared with ISB/GA group.
Clinical index for stress responses
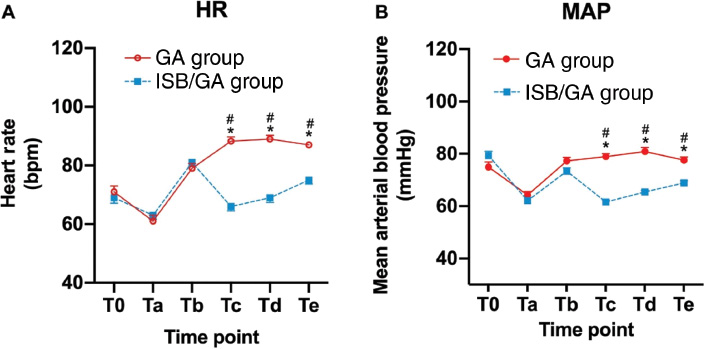
Hemodynamic parameters, such as blood pressure and HR, are the most direct and important clinical index for stress response. We monitored the MAP and HR during the whole surgery and collected the data at six time points: before anesthesia induction (baseline, T0); before tracheal intubation (Ta); after tracheal intubation (Tb); incision (Tc); arthroscopy insertion (Td); tracheal extubation (Te). The result showed that the HR and MAP were more stable in the ISB/GA group than that in the GA group. The MAP (ISB/GA group vs. GA group: T0, 79.3 vs. 74.9 mmHg; Ta, 62.2 vs. 64.3 mmHg; Tb, 73.4 vs. 77.3 mmHg; Tc, 61.6 vs. 78.9 mmHg; Td, 65.4 vs. 80.9 mmHg; Te, 68.9 vs. 77.6 mmHg) and HR (ISB/GA group vs. GA group: T0, 69 vs. 71 bpm; Ta, 63 vs. 61 bpm; Tb, 81 vs. 79 bpm; Tc, 66 vs. 88 bpm; Td, 69 vs. 89 bpm; Te, 75 vs. 87 bpm) were significantly lower in the ISB/GA group at incision, arthroscopy insertion, and tracheal extubation than those in the GA group, and no significant differences were observed at baseline, before intubation, or after intubation (Figure 3).
Figure 3 Hemodynamic changes during operation. (A) Heart rate (HR); (B) Mean arterial blood pressure (MAP). ISB/GA: brachial plexus block in combination with general anesthesia; GA: general anesthesia group. T0 = baseline, Ta = before intubation, Tb = after intubation, Tc = incision, Td = arthroscopy insertion, Te = extubation. Data are expressed as mean ± SEM. *P < 0.05 compared with T0; #P < 0.05 compared with ISB/GA group.
Plasma inflammatory factors analysis
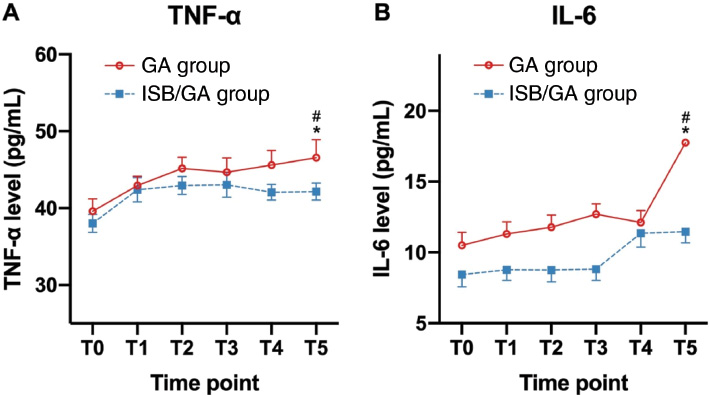
We analyzed the TNF-α and IL-6 levels to determine the inflammatory responses. The TNF-α and IL-6 levels were similar at baseline (ISB/GA group vs. GA group: TNF-α, 38.03 vs. 39.59 pg/mL; IL-6, 8.44 vs. 10.50 pg/mL; no statistical significance between the two groups). We observed that the TNF-α (ISB/GA group vs. GA group: T1, 42.39 vs. 42.94 pg/mL; T2, 42.94 vs. 45.17 pg/mL; T3, 43.05 vs. 44.68 pg/mL; T4, 42.07 vs. 45.61 pg/mL; T5, 42.17 vs. 46.57 pg/mL) and IL-6 (ISB/GA group vs. GA group: T1, 8.78 vs. 11.31 pg/mL; T2, 8,76 vs. 11.78 pg/mL; T3, 8.82 vs. 12.70 pg/mL; T4, 11.36 vs. 12.12 pg/mL; T5, 11.47 vs. 17.76 pg/mL) levels in the GA group are significantly elevated than that those in the ISB/GA group at 6 hours after surgery (Figure 4). The results indicated that ISB/GA could significantly attenuate inflammatory response compared with GA only.
Figure 4 Perioperative plasma inflammatory factors between ISB/GA and GA. (A) Plasma levels of cytokines tumor necrosis factor-α (TNF-α); (B) Plasma levels of interleukin (IL)-6. ISB/GA: brachial plexus block combined general anesthesia; GA: general anesthesia. T0 = baseline; T1 = 5 min after arthroscopy insertion; T2 = 1 hour after the beginning of surgery; T3 = time of wound closure; T4 = 1 hour after surgery; T5 = 6 hours after surgery. Data are expressed as mean ± SEM. *P < 0.05 compared with T0; #P < 0.05 compared with ISB/GA group.
Perioperative consumption of analgesics and recovery profiles
In this study, we used the rapid-effect opioid remifentanil during operation and fentanyl as analgesic after operation to provide analgesia and reduce stress response. The consumptions of remifentanil and fentanyl were significantly lower in the ISB/GA group than that in the GA group (ISB/GA group vs. GA group: fentanyl, 1.13±0.25 vs. 1.93±0.37 mg; remifentanil, 0.21±0.11 vs. 1.31±0.39 mg; Table 2), indicating that ISB/GA provided a significant effect on analgesia that could significantly reduce opioid consumption compared with GA alone.
Table 2 Dose of Opioids and Recovery Profiles during Emergence Period
| GA Group | ISB/GA Group | P | |
|---|---|---|---|
| Average dose of remifentanil (mg)a | 1.31±0.39 | 0.21±0.11 | ≤0.001 |
| Time to eye opening (min)a | 14.1±2.6 | 7.8±1.4 | ≤0.001 |
| Time to extubation (min)a | 15.2±2.7 | 8.9±1.5 | ≤0.001 |
| Patients with agitation, n (%)b | 8 (26.7) | 2 (6.7) | 0.038 |
| VAS score (24 hours)a | 2.1±0.8 | 0.8±0.7 | ≤0.001 |
| Average dose of fentanyl (mg)a | 1.93±0.37 | 1.13±0.25 | ≤0.001 |
Values are means±SD or number. GA: general anesthesia; ISB/GA: brachial plexus block in combination with general anesthesia. aPerformed with t-test; bPerformed with χ2 test.
The recovery profiles were accessed according to the duration from the termination period of sevoflurane to eye opening or extubation. The time courses of eye opening (ISB/GA group vs. GA group, 7.8±1.4 vs. 14.1±2.6 min) and extubation (ISB/GA group vs. GA group, 8.9±1.5 vs. 15.2±2.7 min) were significantly shorter in the ISB/GA group than that in the GA group. Compared with the GA group, the incidence of agitation was less in the ISB/GA group (ISB/GA group vs. GA group, 6.7% vs. 126.7%). The VAS score was also significantly lower at 24 hours after surgery in the ISB/GA group than that in the GA group (ISB/GA group vs. GA group, 0.8±0.7 vs. 2.1±0.8). These results showed that ISB/GA is more beneficial than GA for arthroscopic shoulder surgery in pain release and anesthesia recovery.
Discussion
In this study, we compared GA with or without ISB for arthroscopic shoulder surgery. We found that epinephrine and norepinephrine, the two intrinsic catecholamines, were dramatically increased during operation in the GA group and were significantly higher at the same time points than those in the ISB/GA group. The glucose and lactate plasma levels were also elevated secondary to the increment of epinephrine and norepinephrine. Moreover, we observed that the TNF-α and IL-6 levels were significantly higher in the GA group than those in the ISB/GA group. These data indicate that ISB/GA provided more stress and inflammation inhibition in arthroscopic shoulder surgery. Consistent with previous study, our results demonstrated that ISB/GA brings advantages for the surgery and recovery.
Our data showed that ISB/GA inhibit epinephrine and norepinephrine elevation from 5 min after arthroscopy insertion until 6 hours after surgery. Epinephrine and norepinephrine are the most important stress factors and respond to stimuli early. The stress responses to surgical injury could initiate a cascade of various metabolic and physiologic events through direct activation of both somatic and sympathetic nervous systems [12]. Interestingly, a study by Li et al. indicated that the catecholamine level does not significantly increase in patients receiving GA in combination with epidural anesthesia [13]. The study of Li et al. showed that epidural analgesia for nephrectomy decreases the plasma cortisol level. The authors concluded that epidural blockade inhibits or attenuates surgery-relative stress by blocking afferent neural stimuli from reaching the central nervous system so as to inhibit the efferent activation of the sympathetic nervous system [14]. As a regional anesthesia, brachial plexus block has the similar mechanism with epidural anesthesia, i.e., it prevents afferent neural stimuli from reaching the central nervous system. The epinephrine and norepinephrine levels in the ISB/GA group were stable throughout surgery. As we know, stress induced by stimuli or injury can cause elevated HR and blood pressure. In this study, we found that the HR and MAP of patients in the ISB/GA group were more stable than those in the GA group. This is consistent with the trend of epinephrine and norepinephrine alteration.
It has been proven that elevated catecholamine levels after surgical invasive can also lead to an elevation in blood glucose level through glycogenesis and gluconeogenesis [15]. In this study, we found that glucose was increased following an increase in catecholamine. However, glucose was not significantly different at 5 min after arthroscopy insertion. Surgery is one of the strongest stimuli for cortisol and catecholamine secretions, and the plasma concentration of these hormones increases in a short time (usually within minutes) following the beginning of the surgery [15], and glucose elevation is secondary to the increase in catecholamine, which may provide the explanation. In this study, lactate values were significantly increased during operation in both groups, and the lactate values were significantly higher in the GA group than those in the ISB/GA group. This may also be attributed to stress hyperlactatemia [16].
In our study, although plasma TNF-α and IL-6 concentration gradually increased during and after operation in both groups, the TNF-α and IL-6 levels in the GA group were significantly higher than that in the ISB/GA group at 6 hours after operation indicating a significant inhibition of inflammatory response in the ISB/GA group. A number of clinical studies have demonstrated that following trauma (i.e., surgery), acute-phase pro-inflammatory cytokines would be activated, and the most pivotal cytokines in the situation are TNF-α and IL-6 [17, 18]. TNF-α is one of earliest responder to surgical trauma, whereas IL-6 is one of the key stimuli for acute-phase immunologic responses. The plasma concentration of IL-6 was found to be related to the severity of tissue injury [19].
Regional anesthesia provides an alternative model for analgesia and suppression of stress responses compared to traditional opioids. In our study, ISB can significantly reduce intraoperative opioid dose. Because of its dose dependence, opioid addiction and abuse are becoming an emerging problem [20, 21]. Lehmann et al. have reported that brachial plexus block had the benefit of opioid sparing, thus reducing opioid-related adverse effects [4]. This is consistent with our results. In addition, due to the analgesic effect of brachial plexus block, the concentration of sevoflurane can be much lower than in that in GA, which led to the significantly shorter time to open eyes and tracheal extubation in the ISB/GA group. Meanwhile, the number of patients with agitation also significantly differed between the two groups. Fewer patients were agitated during the period of open eyes and tracheal extubation in the ISB/GA group.
However, we do acknowledge that our study has several limitations. First, this is a single-blinded study, as the participants in the ISB/GA group received an obvious puncture before anesthesia administration. Although the lack of blinding may not affect the primary outcomes of the stress and inflammatory responses, it can possibly influence the clinical assessment based on personal bias. Second, this is a short-term study, and we did not assess long-term functional outcomes, such as nerve injury, because it requires a longer time frame and larger study population. Third, this is a single-center study, and the limited number of participants reduced the power of this study. Further multiple-center study that includes more participants may be beneficial for providing stronger evidence.
In conclusion, compared with GA only, ISB/GA can attenuate surgical stress and inflammatory response and reduce opioid dosage for arthroscopic shoulder surgery, with effects of stable hemodynamic parameters and reduced analgesic consumption postoperatively.
References
- Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy 1993;9:295-300. [PMID: 8323615 DOI: 10.1016/s0749-8063(05)80425-6]
- Arciero RA, Taylor DC, Harrison SA, Snyder RJ, Leahy KE, et al. Interscalene anesthesia for shoulder arthroscopy in a community-sized military hospital. Arthroscopy 1996;12:715-9. [PMID: 9115561 DOI: 10.1016/s0749-8063(96)90176-0]
- Saeki N, Kawamoto M. Tracheal obstruction caused by fluid extravasation during shoulder arthroscopy. Anaesth Intensive Care 2011;39:317-8. [PMID: 21485693]
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol 2015;25:255-61. [PMID: 24829053 DOI: 10.1007/s00590-014-1483-3]
- Ozturk L, Kesimci E, Albayrak T, Kanbak O. Bispectral index-guided general anaesthesia in combination with interscalene block reduces desflurane consumption in arthroscopic shoulder surgery: a clinical comparison of bupivacaine versus levobupivacaine. BMC Anesthesiol 2015;15:104. [PMID: 26194656 DOI: 10.1186/s12871-015-0087-8]
- Yan S, Zhao Y, Zhang H. Efficacy and safety of interscalene block combined with general anesthesia for arthroscopic shoulder surgery: a meta-analysis. J Clin Anesth 2018;47:74-9. [PMID: 29625334 DOI: 10.1016/j.jclinane.2018.03.008]
- Iwasaki M, Edmondson M, Sakamoto A, Ma D. Anesthesia, surgical stress, and “long-term” outcomes. Acta Anaesthesiol Taiwan 2015;53:99-104. [PMID: 26235899 DOI: 10.1016/j.aat.2015.07.002]
- Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 2010;145:858-64. [PMID: 20855756 DOI: 10.1001/archsurg.2010.179]
- Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A 2005;102:5808-13. [PMID: 15817686 DOI: 10.1073/pnas.0501650102]
- Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013;27:669-84. [PMID: 24292568]
- Saglik Y, Yazicioglu D, Cicekler O, Gumus H. Investigation of effects of epidural anaesthesia combined with general anaesthesia on the stress response in patients undergoing hip and knee arthroplasty. Turk J Anaesthesiol Reanim 2015;43:154-61. [PMID: 27366488 DOI: 10.5152/TJAR.2015.26818]
- Oremus K, Safaric Z. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 2004;240:561-2; author reply 2. [PMID: 15319733 DOI: 10.1097/01.sla.0000138626.15285.97]
- Aono H, Takeda A, Tarver SD, Goto H. Stress responses in three different anesthetic techniques for carbon dioxide laparoscopic cholecystectomy. J Clin Anesth 1998;10:546-50. [PMID: 9805694 DOI: 10.1016/s0952-8180(98)00079-8]
- Li Y, Zhu S, Yan M. Combined general/epidural anesthesia (ropivacaine 0.375%) versus general anesthesia for upper abdominal surgery. Anesth Analg 2008;106:1562-5, table of contents. [PMID: 18420877 DOI: 10.1213/ane.0b013e31816d1976]
- Walter M, Rogalla P, Spies C, Kox WJ, Volk T. [Intrathecal misplacement of an interscalene plexus catheter]. Anaesthesist 2005;54:215-9. [PMID: 15599489 DOI: 10.1007/s00101-004-0792-z]
- Garcia-Alvarez M, Marik P, Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol 2014;2:339-47. [PMID: 24703052 DOI: 10.1016/S2213-8587(13)70154-2]
- Hadimioglu N, Ulugol H, Akbas H, Coskunfirat N, Ertug Z, et al. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplant Proc 2012;44:2949-54. [PMID: 23195004 DOI: 10.1016/j.transproceed.2012.08.004]
- Han C, Ding Z, Fan J, Sun J, Qian Y. Comparison of the stress response in patients undergoing gynecological laparoscopic surgery using carbon dioxide pneumoperitoneum or abdominal wall-lifting methods. J Laparoendosc Adv Surg Tech A 2012;22:330-5. [PMID: 22423956 DOI: 10.1089/lap.2011.0412]
- Cruickshank AM, Fraser WD, Burns HJ, van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990;79:161-5. [PMID: 2167805 DOI: 10.1042/cs0790161]
- Kuczynska K, Grzonkowski P, Kacprzak L, Zawilska JB. Abuse of fentanyl: an emerging problem to face. Forensic Sci Int 2018;289:207-14. [PMID: 29902699 DOI: 10.1016/j.forsciint.2018.05.042]
- Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, et al. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med (Lond) 2018;7:16. [PMID: 29988696 DOI: 10.1186/s13741-018-0097-4]